How to submit jobs to clusters
Anton
- mv step5_md-1.gro md.gro
- run prep1-3.sh, you will get out.dms
- run step1-3.sh, job will be submitted
Notes:
- Anton uses kcal/mol/A
- 1.2 was used to do position restraints, ~500 in Gromacs
- ff99SB-starIDLN is self-contained
- ff14SB only has protein, used OL15 for DNA, OL3 for RNA, TIP3P for water, ions.amber1234lm_anton.tip3p for ions
To continue a simulation upto timestep X, simply
anton2 submit jobid --cfg anton.tune.last_time=500000
Most updated force-fields
$ garden with -m viparr/4.7.12c7/bin -m viparr-ffpublic/1.0.3c7/data — viparr —help
usage: viparr input.dms output.dms [ options ]
VIPARR version 4.7.12
VIPARR_FFPATH: /gdn/centos7/0001/x3/prefixes/viparr-ffpublic/1.0.3c7__e02630aad12e/ff
All available forcefields ————————————— aa.amber.ff03r1 — Amber ff03r1 protein forcefield aa.amber.ff14SB — Amber ff14SB protein parameters aa.amber.ff15fb — Amber ff15fb protein parameters aa.amber.ff15ipq — Amber ff15ipq protein parameters aa.amber.ff94 — Amber ff94 protein forcefield aa.amber.ff96 — Amber ff96 protein forcefield aa.amber.ff99 — Amber ff99 protein forcefield aa.amber.ff99SB — Amber ff99SB protein forcefield aa.amber.ff99SB-ILDN — Amber ff99SB-ILDN protein forcefield aa.amber.ff99SB-disp — Amber ff99SB-ILDN protein forcefield with additional vdw and torsion modifications. For use with water.tip4pd-1.6 model aa.amber.ff99SBnmr — Amber ff99SBnmr protein forcefield aa.amber.ff99SBstar-ILDN — Amber ff99SB*-ILDN protein forcefield aa.amber.ffncaa — Amber protein forcefield for 147 non-canonical amino acids aa.amber.ffptm — Amber protein parameters for 32 common post-translational modifications aa.amber.phosaa10 — Amber phosphorylated amino acids aa.charmm.c22 — Charmm22 protein forcefield aa.charmm.c22der — Charmm22 protein forcefield with modified DER side chain charges aa.charmm.c22h — Charmm22 forcefield with pseudopolarization term aa.charmm.c22nocmap — Original charmm22 protein forcefield without added cmap terms aa.charmm.c22star — Charmm22 protein forcefield with backbone and sidechain torsion and DER charge modifications aa.charmm.c36 — Charmm36 protein forcefield aa.charmm.c36m — Charmm36 protein forcefield (c36 with additional cmap and NBFIX modification) carb.charmm.c36 — Charmm36 carbohydrate forcefield ethers.charmm.c35 — Charmm35 ether forcefield ions.amber1234lm_anton.spce — Amber mon- di- tri- and tetra- valent ion parameters for use with spce. Stable on Anton ions.amber1234lm_anton.tip3p — Amber mon- di- tri- and tetra- valent ion parameters for use with tip3p. Stable on Anton ions.amber1234lm_anton.tip4pew — Amber mon- di- tri- and tetra- valent ion parameters for use with tip4pew. Stable on Anton ions.amber1ff99.tip3p — Original Amber ion parameters contained in the ff99 forcefield ions.amber1jc.spce — Amber monovalent ion parameters for use with spce ions.amber1jc.tip3p — Amber monovalent ion parameters for use with tip3p ions.amber1jc.tip4pew — Amber monovalent ion parameters for use with tip4pew ions.amber1lm_iod.all — Amber monovalent ion parameters ions.charmm22 — Original ion parameters used with charmm forcefields. ions.charmm36 — Ion parameters used with charmm36 forcefields. Includes NBFIX terms lipid.amber.lipid14 — Amber Lipid14 parameters lipid.amber.lipid17 — Amber Lipid17 parameters lipid.charmm.c36 — Charmm36 lipid forcefield misc.charmm.all36 — collecton of most charmm36 templates and parameters in one spot na.amber.OL15 — Amber DNA parameters, OL15 variant na.amber.OL3 — Zgarbova et al. (2011) JCTC 7: 2886 na.amber.ROC — Amber RNA parameters, ROC variant na.amber.YIL — Amber RNA parameters, YIL variant na.amber.bsc1 — Amber DNA parameters, bsc1 variant na.charmm.c36 — Charmm36 nucleic acid forcefield water.spc — spc 3-site water model water.spc_opls — spc 3-site water model with geometric combining rules for compatability with opls based forcefields water.spce — spce 3-site water model water.tip3p — tip3p 3-site water model water.tip3p-fb — tip3p-fb 3-site water model water.tip3p_charmm — tip3p 3-site water model with additional hydrogen vdw parameters. Used by charmm water.tip4p — tip4p 4-site water model water.tip4p2005 — tip4p-2005 4-site water model water.tip4pd — tip4pd 4-site water model water.tip4pd-1.6 — tip4pd-1.6 water model, for use with aa.amber-disp water.tip4pew — tip4pew 4-site water model water.tip5p — tip5p 5-site water model
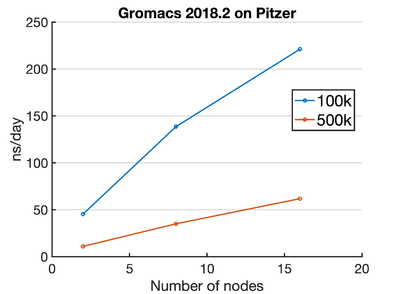
Pitzer
We participated in the Pitzer Early Program, which will become open to the public from Dec 4 2018.
Here is a page on migrating your job from Owens to Pitzer.
Introduction to Pitzer queues and batch limits
Here is a standard Pitzer Gromacs job script:
#PBS -N Ups1-NN
#PBS -l nodes=10:ppn=40
#PBS -l walltime=24:00:00
#PBS -S /bin/bash
#PBS -j oe
#PBS -A PAS1326
trap "cd $PBS_O_WORKDIR; rsync -avh $TMPDIR/ .; exit" TERM
date
module load gromacs
# PBS_O_WORKDIR refers to the directory from which the job was submitted.
cd $PBS_O_WORKDIR
prefix=step5_md
gmx convert-tpr -s ${prefix}-MM.tpr -o ${prefix}-NN.tpr -extend 200000
pbsdcp -p ${prefix}-MM.cpt ${prefix}-NN.tpr $TMPDIR
# Use TMPDIR for best performance.
cd $TMPDIR
mpiexec gmx_mpi mdrun -v -noappend -ntomp 1 -cpi ${prefix}-MM.cpt -deffnm ${prefix}-NN
pbsdcp -p * $PBS_O_WORKDIR/
Things to note:
- The trap command is for copying out files when terminating
- -noappend is required for Gromacs/2018.2 which is default on Pitzer
- -ntomp 1 is A MUST on both Owens and Pitzer, might not be stated on the software page but confirmed by the OSC staff
Submit dependent jobs using this
Benchmarks
| (ns/day) | Amber16 | Gromacs 2019 | Gromacs 2020 | NAMD 2.13 |
|---|---|---|---|---|
| V100 | ||||
| 90-92k | 184.9 | 58.0 | ||
| 400k | 53.4 | 36.8 [1] | 13.5 [1] | |
| 1M | 15.2 [2] | 3.8 [1] | 10.8 [1] | 6.0 |
| RTX 2080 Ti | ||||
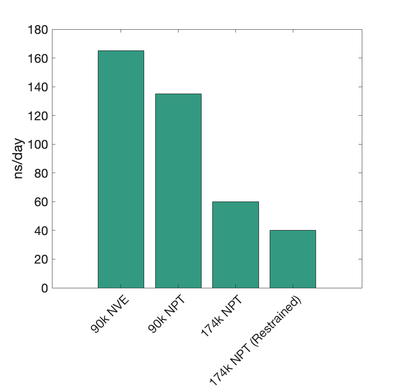
| 90-92k | 137.0 | |||
| 170k | 60.0 | |||
| GTX 1080 | ||||
| 90-92k | ||||
| 170k | 26.1 | 27.3 | ||
[1] 4 x V100 SXM2 16GB
[2] HMR 4fs
Exxact
1 GPU (NVIDIA RTX 2080 Ti)